A third-party submission under 37 CFR 1.290 is filed on its date of receipt in the Office as set forth in 37 CFR 1.6. The holiday/weekend rule set forth in 37 CFR 1.7(a) applies to a third-party submission under 37 CFR 1.290. For example, if the day prior to the date that is six months after publication of an application which has not been allowed but which application was subject to a first Office action including a rejection of at least one claim more than six month previously is a Saturday, the submission may be timely filed on the next business day, e.g., the following Monday via Priority Mail Express® service pursuant to 37 CFR 1.10, hand delivery or preferably via the Office’s dedicated Web-based interface for preissuance submissions. See also Subsection IV.D. below (providing that the certificate of mailing and transmission provisions of 37 CFR 1.8 do not apply, but the United States Postal Service (USPS) Priority Mail Express® service provisions of 37 CFR 1.10 do apply to a third-party submission under 37 CFR 1.290.
37 CFR 1.290(d) identifies the required content of a third-party submission as follows:
37 CFR 1.290(d)(1) provides that any third-party submission under 37 CFR 1.290 must include a document list identifying the documents, or portions of documents, being submitted in accordance with 37 CFR 1.290(e). 37 CFR 1.290(e) sets forth the requirements for identifying the items in the 37 CFR 1.290(d)(1) document list. Because 37 CFR 1.290(d)(1) provides for an item identified in the document list to be either an entire document or a portion of a document, in the case where a lengthy document contains both information of potential relevance to the examination of the application and other information that is not of potential relevance, a third party may choose to identify only the relevant portion of the document (e.g., one chapter of a textbook) in lieu of the entire document where it is practical to do so. Otherwise, the third party should identify the entire document.
When filing in paper, third parties may use form PTO/SB/429 (or equivalent) to prepare the document list in accordance with 37 CFR 1.290(d)(1) and 37 CFR 1.290(e). Electronic filing via the Office’s dedicated Web-based interface for preissuance submissions in EFS-Web is an alternative to paper filing using form PTO/SB/429 (or equivalent). Use of this form will not be necessary for third-party submissions filed electronically via the Office’s dedicated Web-based interface for preissuance submissions, as this interface will prompt the third party to complete the fields that are provided on the form and will automatically format the entered information into an electronic version of the form PTO/SB/429 for electronic submission. While use of form PTO/SB/429 is not required for paper submissions, form PTO/SB/429 is designed to help ensure that important requirements are not overlooked, such as the document listing requirements pursuant to 37 CFR 1.290(e) and the required statements pursuant to 37 CFR 1.290(d)(5). The form PTO/SB/429 also enables the third party to indicate whether a fee is due or to select the "first and only" statement pursuant to 37 CFR 1.290(g) where the fee exemption applies. Form PTO/SB/429 and instructions for completion are available on the USPTO website at www.uspto.gov/forms.
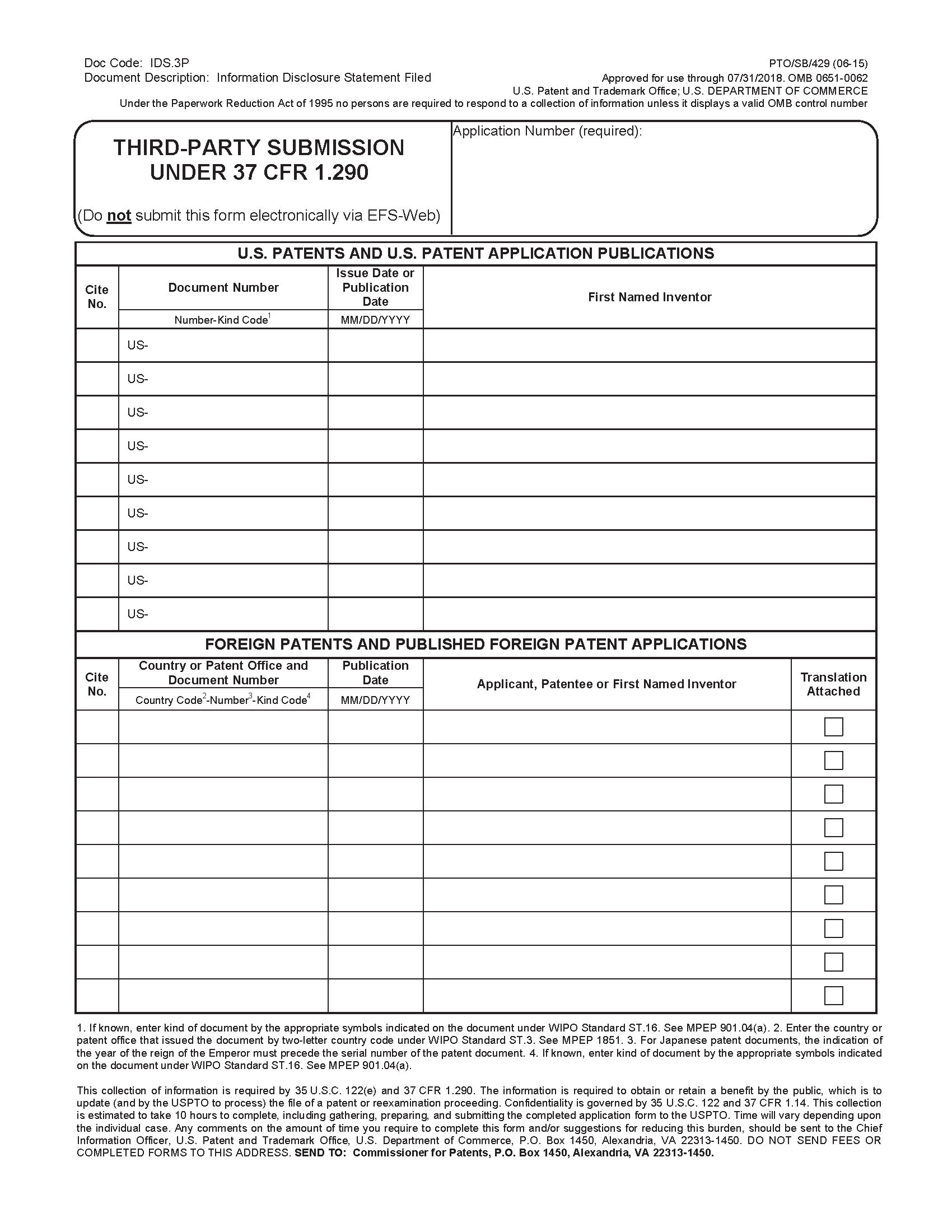

37 CFR 1.290(e) sets forth the requirements for identifying the items in the document list pursuant to 37 CFR 1.290(d)(1). Section 1.290(e) requires the document list include a heading that identifies the list as a third-party submission under 37 CFR 1.290. 37 CFR 1.290(e) also requires that the document list identify on each page of the list, the application number (i.e., the series code and serial number) of the application in which the submission is being filed. This requirement is consistent with the requirement set forth in 37 CFR 1.98(a)(1)(i) for applicant information disclosure statement listings. 37 CFR 1.290(e) further requires that U.S. patents and U.S. patent application publications be listed in a separate section from other items in the document list. Separating the listing of U.S. patents and U.S. patent application publications from the listing of other items in the document list will facilitate printing the U.S. patents and U.S. patent application publications considered by the examiner in a third-party submission on the face of the patent. The dedicated Web-based interface for electronically filing preissuance submissions will automatically generate a document list in accordance with these requirements of 37 CFR 1.290(e).
Sections 1.290(e)(1) through (e)(4) set forth the requirements for identifying the items in the 37 CFR 1.290(d)(1) document list.
37 CFR 1.290(e)(1) requires that each U.S. patent be identified by patent number, first named inventor, and issue date. 37 CFR 1.290(e)(2) requires that each U.S. patent application publication be identified by patent application publication number, first named inventor, and publication date.
37 CFR 1.290(e)(3) requires that each foreign patent or published foreign patent application be identified by the country or patent office that issued the patent or published the application; the applicant, patentee, or first named inventor; an appropriate document number; and the publication date indicated on the patent or published application. The requirement for U.S. patents and patent application publications to be identified by first named inventor, and for foreign patents and published patent applications to be identified by the applicant, patentee, or first named inventor, is intended to aid in identifying the items in the document list in the event the application number, publication number, or other appropriate document number data is in error, for example, inadvertently transposed. Further, 37 CFR 1.290(e)(3) offers flexibility in permitting identification of foreign patents and published foreign patent applications by expanding the identification to also include the applicant or patentee, in addition to the first named inventor.
All non-patent publications, such as Office actions, journal articles, communications from foreign patent offices, court documents, etc. that qualify as publications should be listed under the "Non-Patent Publications" section of the form PTO/SB/429 (or equivalent) or entered in the "Non-Patent Publications" section of the Office’s dedicated Web-based interface for preissuance submissions when filing electronically.
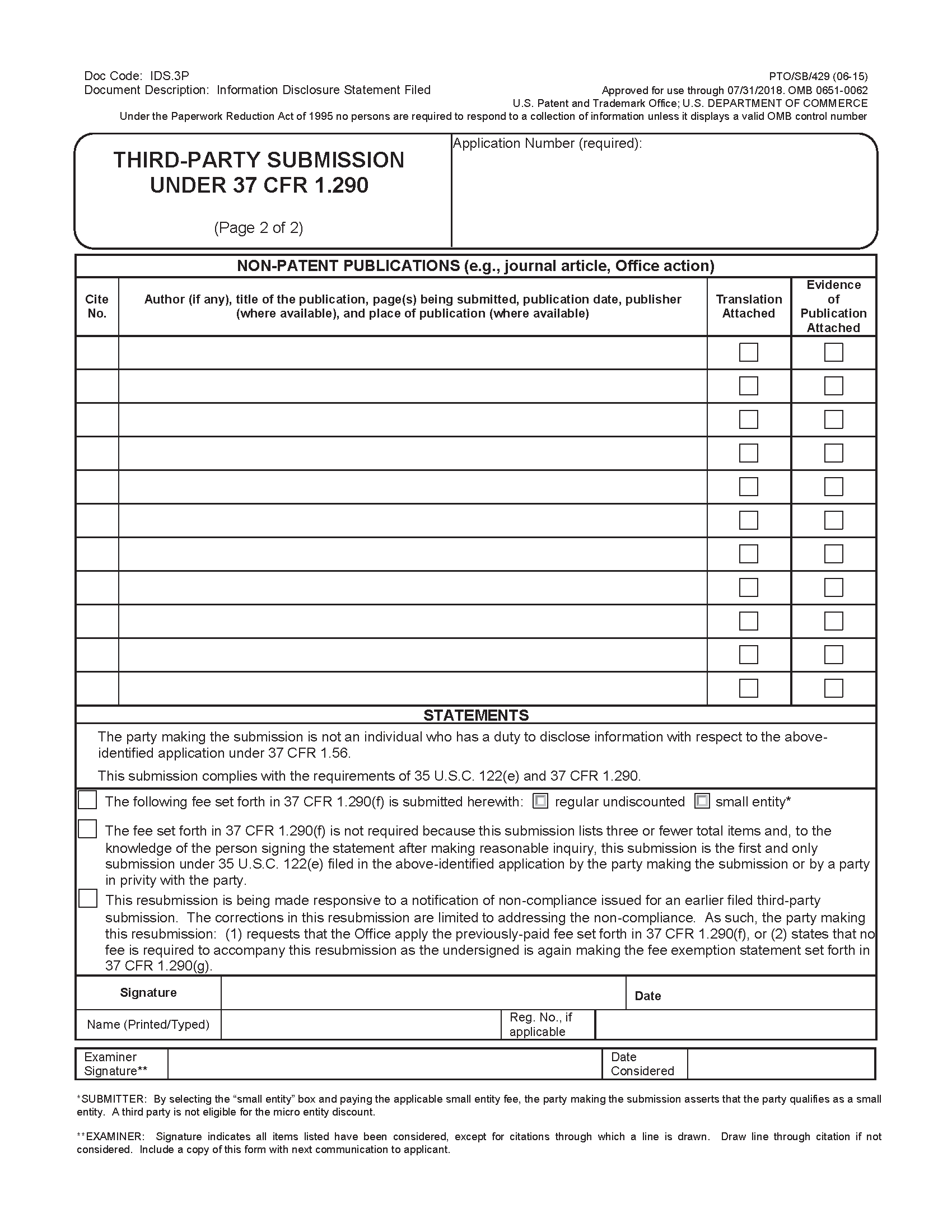
37 CFR 1.290(e)(4) requires that each non-patent publication be identified by author (if any), title, pages being submitted, publication date, and where available, publisher and place of publication. However, 37 CFR 1.290(e)(4) does not preclude a third party from providing additional information not specified in 37 CFR 1.290(e)(4) (e.g., journal title and volume/issue information for a journal article). Because publisher and place of publication information may not be available in some instances, 37 CFR 1.290(e)(4) emphasizes that such information need only be provided where it is available. For publications obtained from the Internet, the uniform resource locator (URL) of the Web page that is the source of the publication must be provided for the place of publication (e.g., "www.uspto.gov"). Further, for an Internet publication obtained from a website that archives Web pages, both the URL of the archived Web page submitted for consideration and the URL of the website from which the archived copy of the Web page was obtained should be provided on the document listing (e.g., "Hand Tools," Web page <http://www.farmshopstore.com/handtools.html>, 1 page, August 18, 2009, retrieved from Internet Archive Wayback Machine <http://web.archive.org/web/20090818144217/ http://www.farmshopstore.com/handtools.html> on December 20, 2012).
37 CFR 1.290(e)(4) further requires that, if no publication date is known, the third party must provide evidence of publication. This requirement recognizes that some documents may not indicate a date of publication. Where the actual publication date of a non-patent document is not known, a third party must, at a minimum, provide a date of retrieval (e.g., the date a Web page was retrieved) or a time frame (e.g., a year, a month and year, a certain period of time) when the document was available as a publication for purposes of identifying the document by publication date pursuant to 37 CFR 1.290(e)(4), in addition to including evidence that establishes the document as a publication. See Subsection III.A. below for additional discussion regarding evidence of publication.
37 CFR 1.290(d)(2) requires a concise description of the asserted relevance of each item identified in the document list in view of the statutory requirement of 35 U.S.C. 122(e)(2)(A) that each third-party preissuance submission be accompanied by a "concise description of the asserted relevance of each submitted document." A concise description of relevance for an item is a statement of facts regarding the submitted evidence (i.e., the patent, published patent application, or other publication) and will not, itself, be treated as evidence. The concise description should set forth facts, explaining how an item listed is of potential relevance to the examination of the application in which the third-party submission has been filed.
The concise description of relevance for a listed publication can be presented in any format that would best explain to the examiner the relevance of the accompanying document, such as in a narrative description or a claim chart. A concise description of relevance is most effective when it draws the examiner’s attention to the potential relevance of a submitted document to the examination of an application. A concise description that points out the relevant pages or lines of the respective document may be an effective way to draw the examiner’s attention to the potential relevance of the document, particularly where the document is lengthy and complex and the third party can identify a highly relevant section, such as a particular figure or paragraph.
A third party using the Office’s dedicated Web-based interface to electronically file a third-party submission may fill in the concise description of relevance field for an item or upload a separate paper with the concise description for the item in lieu of entering the concise description in the field. See Subsection IV.E. below for more information regarding electronic filing. When filing in paper, a third party should provide the concise description of relevance for an item as a separate paper (as opposed to combining the concise descriptions of relevance for all items into a single paper). Providing, for each concise description of relevance, a separate paper that prominently identifies the item in the document list to which the concise description pertains will help ensure that the screener and the examiner can readily identify it. See Subsection IV.F below for more information regarding paper filing.
At a minimum, a concise description of relevance must be more than a bare statement that the document is relevant because such a statement does not amount to a meaningful concise description. For example, the following statements, presented alone, would not be considered anything more than bare statements of relevance that do not rise to the level of meaningful concise descriptions: "Document 1 is relevant," "See Document 1," "Document 1 discloses/may disclose the invention," and "Document 1 teaches the invention in Claim 1." Additionally, a copy of the listed document that is merely annotated or highlighted will not be deemed a proper concise description of relevance. Further, concise descriptions of relevance that appear to be mere form paragraphs/letters in opposition to a general class of invention or technology will not be deemed proper concise descriptions of relevance.
While there is no page limit on a concise description of relevance, third parties should refrain from submitting a verbose description of relevance, not only because the statute calls for a "concise" description, but also because a focused description is more effective in drawing the examiner’s attention to the relevant issues. For example, a description that includes an introductory paragraph describing the field of technology of a document and a claim chart that maps portions of the document to different claim elements would likely be considered "concise." On the other hand, descriptions that merely repeat in narrative format the same information that is also depicted in a claim chart or that approach the length of the documents themselves will not likely be considered "concise."
Third-party submissions that include unpublished materials as attachments to or inserted into the text of a concise description of relevance of a listed publication will be found to be non-compliant. For example, where a third party submits a publication for consideration, describes how a feature shown in an image from the publication is relevant, and inserts an image from a different source into the concise description to show details that are not visible or otherwise apparent in the published image, such submission would be deemed non-compliant unless the image from the different source was also published and separately listed for consideration.
The statutory requirement for a concise description of relevance should not be interpreted as permitting a third party to participate in the prosecution of an application, as 35 U.S.C. 122(c) prohibits the initiation of a protest or other form of pre-issuance opposition for published applications without the consent of the applicant. Therefore, while a concise description of relevance may include claim charts (i.e., mapping various portions of a submitted document to different claim elements), the concise description of relevance is not an invitation to a third party to propose rejections of the claims or set forth arguments relating to an Office action in the application or to an applicant’s reply to an Office action in the application. Unlike the concise explanation for a protest under 37 CFR 1.291, which allows for arguments against patentability, the concise description of relevance required by 35 U.S.C. 122(e) is limited to a factual description of a document’s relevance. The concise description of relevance, therefore, does not permit third parties to submit arguments against patentability or set forth conclusions regarding whether one or more claims are patentable. In other words, the concise description of relevance must not rise to the level of a protest under 37 CFR 1.291.
Examples of compliant concise descriptions formatted as a narrative:
"Claim 1 recites a refrigeration system comprising elements A, B, and C. Publication X discloses the refrigeration system recited in claim 1, except that the refrigeration system disclosed in publication X uses element D instead of element C. See Figure 1 on page 2 of publication X. Publication Y discloses the specific element C recited in claim 1, but not in the context of refrigeration systems. See pages 1-3 of publication Y. Publication Z teaches that element C is frequently used in refrigeration systems. See lines 2-10 on page 6 of publication Z."
"Claim 1 recites a chemical composition comprising chemicals A, B, C, and D. Patent publication X teaches a chemical composition comprising chemicals A, B, C, and E. See claim 4 of patent publication X. Publication Y teaches chemical D and discusses why chemical D is an art-recognized equivalent of chemical E. See page 4 of publication Y."
Examples of non-compliant concise descriptions formatted as a narrative (the non-compliant portion is shown in bold):
"Claim 1 recites a refrigeration system comprising elements A, B, and C. Publication X discloses the refrigeration system recited in claim 1, except that the refrigeration system disclosed in publication X uses element D instead of element C. See Figure 1 on page 2 of publication X. Publication Y discloses the specific element C recited in claim 1, but not in the context of refrigeration systems. See pages 1-3 of publication Y. Publication Z teaches that element C is frequently used in refrigeration systems. See lines 2-10 on page 6 of publication Z. It would have been obvious to one of ordinary skill in the art to combine the teachings of publication X and publication Y to obtain the refrigeration system recited in claim 1."
"Claim 1 recites a chemical composition comprising chemicals A, B, C, and D. Patent publication X teaches a chemical composition comprising chemicals A, B, C, and E. See claim 4 of patent publication X. Publication Y teaches chemical D and discusses why chemical D is an art-recognized equivalent of chemical E. See pages 3-4 of publication Y. The composition of claim 1 is unpatentable in view of publication X and publication Y."
Examples of compliant concise descriptions formatted as a claim chart for a claim having only two elements:
| Claim 1 | Publication X |
| Preamble | As discussed on page 1, publication X discloses a machine that performs the same function as the machine recited in claim 1. The machine set forth in publication X includes many of the same parts discussed in the specification of this application. |
| Element A | For example, in the first embodiment depicted in Figure 2 and discussed on page 5, the machine of publication X expressly includes element A of claim 1. See lines 7-14 on page 5 of publication X. |
| Element B | The first embodiment also includes element B of claim 1. See lines 1-3 on page 6 of publication X. |
| Claim 1 | Publication X | Publication Y |
| Preamble | Publication X discloses a machine that performs the same function as the machine recited in claim 1. The machine set forth in publication X includes many of the same parts discussed in the specification of this application. | Publication Y discloses a machine that performs the same function as the machine recited in claim 1. |
| Element A | For example, in the first embodiment depicted in Figure 2 and discussed on page 5, the machine of publication X expressly includes element A of claim 1. See lines 7-14 on page 5 of publication X. | |
| Element B | Publication Y teaches a machine having element B of claim 1. See lines 1-3 on page 6 of publication Y. Publication Y teaches the benefits of using element B in this type of a machine. |
Examples of non-compliant concise descriptions formatted as a claim chart for a claim having only two elements (the non-compliant portion is shown in bold):
| Claim 1 | Publication X |
| Preamble | As discussed on page 1, publication X discloses a machine that performs the same function as the machine recited in claim 1. The machine set forth in publication X includes many of the same parts discussed in the specification of this application. |
| Element A | For example, in the first embodiment depicted in Figure 2 and discussed on page 5, the machine of publication X expressly includes element A of claim 1. See lines 7-14 on page 5 of publication X. |
| Element B | The first embodiment also includes element B of claim 1. See lines 1-3 on page 6 of publication X. Thus, publication X anticipates claim 1 because it teaches all of the elements of claim 1. |
| Claim 1 | Publication X | Publication Y |
| Preamble | Publication X discloses a machine that performs the same function as the machine recited in claim 1. The machine set forth in publication X includes many of the same parts discussed in the specification of this application. | Publication Y discloses a machine that performs the same function as the machine recited in claim 1. |
| Element A | For example, in the first embodiment depicted in Figure 2 and discussed on page 5, the machine of publication X expressly includes element A of claim 1. See lines 7-14 on page 5 of publication X. | |
| Element B | Publication Y teaches a machine having element B of claim 1. See lines 1-3 on page 6 of publication Y. Publication Y teaches the benefits of using element B in this type of a machine. Accordingly, claim 1 is unpatentable in view of the combination of publication X and publication Y. |
A concise description of relevance for a submitted document is not considered evidence but, rather, a statement of facts regarding the submitted evidence. Accordingly, the Office will not consider a declaration as evidence, where such declaration is submitted as a concise description of relevance for a document. Where a third party submits a declaration for the concise description of relevance, the concise description of relevance must not amount to an attempt at third-party participation in the examination of the application.
37 CFR 1.290(d)(3) requires submission of a legible copy of each item identified in the document list, other than U.S. patents and U.S. patent application publications. See 37 CFR 1.98(a)(2)(ii) and MPEP § 609.04(a). Any copies of documents that are submitted in color will be scanned into black and white prior to entry of a compliant submission in the record of an application. There is no provision for the submission of copies of documents via compact disc or other electronic data storage medium. However, a third party may upload electronic copies of documents when using the Office’s dedicated Web-based interface to electronically file a third-party submission. See Subsection IV.E. below.
37 CFR 1.290(d)(1) provides for the listing of either entire documents or portions of documents. Thus, where only a portion of a document is listed as an item in the document list, a copy of that portion and not a copy of the entire document (e.g., where a particular chapter of a book is listed and not the entire book) must be submitted. Further, when a copy of only a portion of a document is submitted, copies of pages of the document that provide identifying information (e.g., a copy of the cover, the title page, the copyright information page, etc.) should also be submitted. Under 37 CFR 1.290(d)(3), copies of U.S. patents and U.S. patent application publications need not be submitted because such documents are readily accessible to examiners.
Whether filing a third-party submission under 37 CFR 1.290 in paper or electronically, it would be a best practice for third parties to include an identifying label for each item in the document list and place the identifying label on the accompanying concise description of relevance for the item, on the copy of the item (if submitted), and on the translation of the item (if submitted) so that screeners and examiners can more quickly identify the descriptions of relevance, copies, and translations that correspond to each item in the document list.
Images of non-patent literature (NPL) cited in a compliant third-party submission will not be available for either viewing or downloading through Patent Center. However, when entering a compliant third-party submission into an application file, the Office will separate the document list from the copies of the documents so that the identifying bibliographical information for the documents cited in the third-party submission will be visible in Patent Center. The Office currently employs such a practice when entering IDS submissions under 37 CFR 1.98.
37 CFR 1.290(d)(4) requires an English language translation of any non-English language item identified in the document list. A translation submitted pursuant to 37 CFR 1.290(d)(4) may be a reliable machine translation and need not be certified. 37 CFR 1.290(d)(1) provides for the listing of either entire documents or portions of documents. Thus, where only a portion of a non-English language document is listed, a translation of the entire non-English language document must not be submitted. Rather, a copy of the listed portion of the non-English language document and a translation of only this portion must be submitted.
37 CFR 1.290(d)(5)(i) requires a statement by the party making the submission that the party is not an individual who has a duty to disclose information with respect to the application (i.e., each individual associated with the filing and prosecution of the patent application) under 37 CFR 1.56. Such statement is intended to avoid potential misuse of third-party submissions by applicants (e.g., by employing a third-party "straw man") to attempt to circumvent the IDS rules. 37 CFR 1.290(d)(5)(ii) requires a statement by the party making the submission that the submission complies with the requirements of 35 U.S.C. 122(e) and 37 CFR 1.290. Additionally, to take advantage of the fee exemption, a third-party submission must be accompanied by the statement under 37 CFR 1.290(g). See Subsection IV.F. below for more information regarding the fee exemption.
To facilitate compliance by third parties, form PTO/SB/429 and the dedicated Web-based interface for preissuance submissions include the statements required by 37 CFR 1.290(d)(5)(i) and (ii), as well as the statement under 37 CFR 1.290(g) (which can be selected if applicable). The Office will not entertain challenges to the accuracy of such statements because, pursuant to 37 CFR 11.18(b), whoever knowingly and willfully makes any false, fictitious, or fraudulent statements or representations to the Office shall be subject to the penalties set forth under 18 U.S.C. 1001. 37 CFR 11.18(b) applies to any paper presented to the Office, whether by a practitioner or non-practitioner.
The Office cannot permit a third-party submission to be presented unsigned by the submitter in view of the signature requirement set forth in 37 CFR 1.4 for papers filed in a patent application, which require a person’s signature. Third-party submissions are required to be signed because 37 CFR 1.290(d)(5) and 37 CFR 1.290(g) (if applicable) require statements by the party making the submission. Thus, a third-party submission must be signed by the submitter, but there is no requirement to identify a real party in interest. A real party in interest can remain anonymous by having someone else make the third-party submission for them, but the submitter cannot remain anonymous.
37 CFR 1.290(f) requires payment of the fee set forth in 37 CFR 1.17(o) for every ten items or fraction thereof listed in the document list, except where the submission is accompanied by the statement set forth in 37 CFR 1.290(g). The Office will determine the item count based on the 37 CFR 1.290(d)(1) document list. Thus, if a U.S. patent or a U.S. patent application publication is identified in the document list, but a copy of the item is not submitted (i.e., because a copy is not required), the listed U.S. patent or U.S. patent application publication will be counted toward the document count. If a copy of an item is submitted but the item is not identified in the document list, the item will not be counted or considered and will be discarded. Additionally, if a third party identifies an item in the 37 CFR 1.290(d)(1) document list that is only a portion of a publication, the portion of the publication will be counted as one item. Further, while a third party is permitted to cite different publications that are all available from the same electronic source, such as a website, each such publication listed will be counted as a separate item. See Changes To Implement the Preissuance Submissions by Third Parties Provision of the Leahy-Smith America Invents Act, 77 Fed. Reg. 42150, 42163 (July 17, 2012) (final rule) for guidance on what constitutes a separate document on a website.
When filing electronically, payment may be made by credit card, USPTO deposit account, or electronic funds transfer and the fee must accompany the submission at the time of filing. Credit card information for electronic credit card payments should be entered exclusively on the USPTO website providing electronic payment capability. When filing in paper, payment may be made by check, money order, credit card, or deposit account. Checks and money orders must be made payable to the Director of the United States Patent and Trademark Office. Credit Card Payment Form (PTO-2038) is available for making payment by credit card for paper submission. See www.uspto.gov/forms. To protect credit card information, form PTO-2038 must not be submitted electronically through EFS-Web.
37 CFR 1.290(g) provides an exemption from the 37 CFR 1.290(f) fee requirement where a third-party submission listing three or fewer total items is the first third-party submission by a third party, or a party in privity with the third party, in a given application. Where one third party takes advantage of the fee exemption in an application, another third party is not precluded from also taking advantage of the fee exemption in the same application as long as the third parties are not in privity with each other.
Third parties are not required to avail themselves of the fee exemption. Thus, a third party can make a first submission of three or fewer documents in an application and choose to pay the fee instead of making the statement under 37 CFR 1.290(g) (e.g., where a third party is uncertain whether it is appropriate to make the "privity" statement pursuant to 37 CFR 1.290(g).)
To implement the fee exemption in 37 CFR 1.290(g) and avoid potential misuse of such exemption, exemption-eligible third-party submissions must be accompanied by a statement of the third party (i.e., "the party making the submission") that, to the knowledge of the person signing the statement after making reasonable inquiry, the submission is the first and only third-party submission in the application by the third party or a party in privity with the third party. To preclude a third party from making multiple third-party submissions in the same application on the same day and asserting that each such submission is the first third-party submission in the application by the third party, the 37 CFR 1.290(g) statement requires that the submission be the "first and only" third-party submission. This statement will not, however, preclude the third party from making more than one third-party submission in an application, where the need for the subsequent submissions was not known at the time the third party filed the earlier submission that included the 37 CFR 1.290(g) statement. The third party would not be required to state in any such subsequent submission that the need for the subsequent submission was not known at the time the third party filed the earlier submission that included the 37 CFR 1.290(g) statement. Any such subsequent submission, however, would not be exempt from the 37 CFR 1.290(f) fee requirement.
Where a third party receives a notification of non-compliance for a third-party submission, the third party may make necessary revisions to its submission, limited to addressing the non-compliance, and resubmit the now corrected submission provided the statutory time period for filing a third-party submission has not closed. The resubmission must be another complete submission, as the Office will not accept amendments to the non-compliant submission. See Subsection II. for content requirements for a third-party submission. To be complete, the appropriate fee for the number of documents being submitted (e.g., $180 for 1-10 documents) must accompany any resubmission made in response to a notification of non-compliance. However, to satisfy the fee requirement for a resubmission after a finding of non-compliance where the proper fee set forth in 37 CFR 1.290(f) accompanied the non-compliant submission, the third party may request that the Office apply the previously-paid fee to the resubmission. Similarly, to satisfy the fee requirement for a resubmission after a finding of non-compliance where the third party’s non-compliant submission of three or fewer documents was accompanied by the fee