The right to a plant patent stems from:
Whoever invents or discovers and asexually reproduces any distinct and new variety of plant, including cultivated sports, mutants, hybrids, and newly found seedlings, other than a tuber propagated plant or a plant found in an uncultivated state, may obtain a patent therefor, subject to the conditions and requirements of this title.
The provisions of this title relating to patents for inventions shall apply to patents for plants, except as otherwise provided.
Asexually propagated plants are those that are reproduced by means other than from seeds, such as by the rooting of cuttings, by layering, budding, grafting, inarching, etc. Plants capable of sexual reproduction are not excluded from consideration if they have also been asexually reproduced.
With reference to tuber propagated plants, for which a plant patent cannot be obtained, the term "tuber" is used in its narrow horticultural sense as meaning a short, thickened portion of an underground branch. Such plants covered by the term "tuber propagated" are the Irish potato and the Jerusalem artichoke. This exception is made because this group alone, among asexually reproduced plants, is propagated by the same part of the plant that is sold as food.
The term "plant" has been interpreted to mean "plant" in the ordinary and accepted sense and not in the strict scientific sense and thus excludes bacteria. In re Arzberger, 112 F. 2d 834, 46 USPQ 32 (CCPA 1940). The term "plant" thus does not include asexual propagating material, per se. Ex parte Hibberd, 227 USPQ 443, 447 (Bd. Pat. App. & Int. 1985).
35 U.S.C. 161 originated as an amendment to the pre-existing patent statute with the Plant Patent Act of 1930. As enacted, the "invents or discovers" requirement limited patent protection to plants "that were created as a result of plant breeding or other agricultural and horticultural efforts and that were created by the inventor." In re Beineke, 690 F.3d 1344, 1352, 103 USPQ2d 1872, 1877 (Fed. Cir. 2012). The plant patent provisions were separated from the utility patent provisions in the Patent Act of 1952 to create 35 U.S.C. 161. Id. at 1348 n.2, 103 USPQ2d 1875 n.2. 35 U.S.C. 161 was amended in 1954 to extend protection to "newly found seedlings," provided they were found in a cultivated state, but did not otherwise alter the scope of plant patent protection. Id. at 1352-53, 103 USPQ2d at 1878-79. In Beineke, the Federal Circuit held that two century-old oak trees found on the lawn of a home were ineligible for patent protection under 35 U.S.C. 161 because they were not created from inception by human activity and created by the inventor (i.e., the patent applicant) as required by the 1930 Act, nor were they "newly found seedlings" under the 1954 amendment. Id. at 1348, 1352, 103 USPQ2d at 1875, 1879.
In reviewing the history of the Plant Patent Act, the Supreme Court explained:
Prior to 1930, two factors were thought to remove plants from patent protection. The first was the belief that plants, even those artificially bred, were products of nature for purposes of the patent law…. The second obstacle to patent protection for plants was the fact that plants were thought not amenable to the "written description" requirement of the patent law. See U.S.C. § 112. *** In enacting the Plant Patent Act, Congress addressed both of these concerns. It explained at length its belief that the work of the plant breeder "in aid of nature" was patentable invention. S. Rep. No. 315, 71st Cong., 2d Sess., 6-8 (1930); H.R. Rep. No. 1129, 71st Cong., 2d Sess., 7-9 (1930). And it relaxed the written description requirement in favor of "a description … as complete as is reasonably possible." 35 U.S.C. § 162.
Diamond v. Chakrabarty, 447 U.S. 303, 311-312 (1980).
An asexually reproduced plant may alternatively be protected under 35 U.S.C. 101, provided the written description requirement can be satisfied. See 35 U.S.C. 112. In J.E.M. Ag Supply, Inc. v. Pioneer Hi-Bred Int’ l, Inc., the Supreme Court held that patentable subject matter under 35 U.S.C. 101 includes newly developed plants, even though plant protection is also available under the Plant Patent Act (35 U.S.C. 161 -164 ) and the Plant Variety Protection Act (7 U.S.C. 2321 et. seq.). J.E.M. Ag Supply, Inc. v. Pioneer Hi-Bred Int’ l, Inc., 534 U.S. 124, 143-46, 122 S.Ct. 593, 605-06, 60 USPQ2d 1865, 1874 (2001) (The scope of coverage of 35 U.S.C. 101 is not limited by the Plant Patent Act or the Plant Variety Protection Act; each statute has different requirements and protections). An application filed under 35 U.S.C. 101 may claim the same asexually reproduced plant that is claimed under 35 U.S.C. 161, as well as plant materials and processes involving plant materials. See MPEP § 2105.
The filing of a terminal disclaimer may be used in appropriate situations to overcome an obviousness-type double patenting rejection based on claims to the asexually reproduced plant and/or fruit and propagating material thereof in an application under 35 U.S.C. 101 and the claim to the same asexually reproduced plant in an application under 35 U.S.C. 161.
In the case of a plant patent, the grant shall include the right to exclude others from asexually reproducing the plant, and from using, offering for sale, or selling the plant so reproduced, or any of its parts, throughout the United States, or from importing the plant so reproduced, or any parts thereof, into the United States.
As provided in 35 U.S.C. 161, the rights associated with a plant patent include the rights associated with a utility patent, and the "right to exclude" has additional terms provided in 35 U.S.C. 163. A plant patent issuing from an application filed after June 7, 1995 has a term which expires 20 years after the filing date of the application, or any earlier filing date claimed under 35 U.S.C. 120, 121 or 365(c) . See MPEP § 2701. Plant patent applications will be published pursuant to 35 U.S.C. 122(b).
The rules relating to applications for patent for other inventions or discoveries are also applicable to applications for patents for plants except as otherwise provided.
*****
*****
An application for a plant patent consists of the same parts as other applications. For information pertaining to the inventor's oath or declaration, specification and claim, or drawings, see MPEP § 1604, 1605, or 1606, respectively.
[Editor Note: Applicable only to patent applications filed under 35 U.S.C. 111(a) or 363 on or after September 16, 2012.]
The inventor named for a plant patent application must be the person who has invented or discovered and asexually reproduced the new and distinct variety of plant for which a patent is sought. The inventor's oath or declaration, in addition to the averments required by § 1.63 or § 1.64, must state that the inventor has asexually reproduced the plant. Where the plant is a newly found plant, the inventor's oath or declaration must also state that it was found in a cultivated area.
[Editor Note: Not applicable to patent applications filed under 35 U.S.C. 111(a) or 363 on or after September 16, 2012.]
The applicant for a plant patent must be the person who has invented or discovered and asexually reproduced the new and distinct variety of plant for which a patent is sought (or as provided in §§ 1.42, 1.43 and 1.47). The oath or declaration required of the applicant, in addition to the averments required by § 1.63, must state that he or she has asexually reproduced the plant. Where the plant is a newly found plant, the oath or declaration must also state that it was found in a cultivated area.
The inventor's oath or declaration, in addition to the averments required by 37 CFR 1.63 or 37 CFR 1.64, (or, for applications filed before September 16, 2012, pre-AIA 37 CFR 1.63 ) must state that the inventor has asexually reproduced the plant. Where the plant is a newly found plant, the inventor's oath or declaration must also state that it was found in a cultivated area.
A Plant Patent Application (35 U.S.C. 161 ) Declaration, Form PTO/AIA/09, may be used to submit a declaration in a plant application filed on or after September 16, 2012.
In an application for a plant patent, there can be joint inventors. See Ex parte Kluis, 70 USPQ 165 (Bd. App. 1945).


No plant patent shall be declared invalid for noncompliance with section 112 if the description is as complete as is reasonably possible.
The claim in the specification shall be in formal terms to the plant shown and described.
The claim shall be in formal terms to the new and distinct variety of the specified plant as described and illustrated, and may also recite the principal distinguishing characteristics. More than one claim is not permitted.
The specification should include a complete detailed description of the plant and the characteristics thereof that distinguish the same over related known varieties, and its antecedents, expressed in botanical terms in the general form followed in standard botanical textbooks or publications dealing with the varieties of the kind of plant involved (evergreen tree, dahlia plant, rose plant, apple tree, etc.), rather than a mere broad nonbotanical characterization such as commonly found in nursery or seed catalogs. The specification should also include the origin or parentage and the genus and species designation of the plant variety sought to be patented. The Latin name of the genus and species of the plant claimed should be stated and preceded by the heading set forth in 37 CFR 1.163(c)(4). The specification must particularly point out where, e.g., location or place of business, and in what manner the variety of plant has been asexually reproduced.
Form Paragraphs 16.01, 16.09, and 16.10 may be used to object to the disclosure under 37 CFR 1.163(a).
The application is objected to under 37 CFR 1.163(a) because the specification does not "particularly point out where and in what manner the variety of plant has been asexually reproduced." Correction is required.
The disclosure is objected to under 37 CFR 1.163(a) because the specification presents less than a full and complete botanical description and the characteristics which distinguish over related known varieties. More specifically: [1].
The disclosure is objected to under 37 CFR 1.163(a) because the specification does not particularly point out the location and character of the area where the plant was discovered.
Where color is a distinctive feature of the plant, the color should be positively identified in the specification by reference to a designated color as given by a recognized color dictionary or color chart.
Form Paragraphs 16.02 and 16.03 may be used to object to the disclosure or reject the claim, respectively, because of a lack of a clear and complete disclosure with regard to colors.
The disclosure is objected to under 35 U.S.C. 112(a) or pre-AIA 35 U.S.C. 112, first paragraph, because the [1] colors specified fail to correspond with those shown.
The claim is rejected under 35 U.S.C. 112(a) or pre-AIA 35 U.S.C. 112, first paragraph, as being unsupported by a clear and complete disclosure with regard to [1] colors, for the following reasons: [2].
If the written description of a plant is deficient in certain respects (see, e.g., In re Greer, 484 F.2d 488, 179 USPQ 301 (CCPA 1973)), a clarification or additional description of the plant, or even a wholesale substitution of the original description so long as not totally inconsistent and unrelated to the original description and photograph of the plant may be submitted in reply to an Office action. Such submission will not constitute new matter under 35 U.S.C. 132. Jessel v. Newland, 195 USPQ 678, 684 (Dep. Comm’r Pat. 1977).
The rules on Deposit of Biological Materials, 37 CFR 1.801 -1.809, do not apply to plant patent applications in view of the reduced disclosure requirements of 35 U.S.C. 162, even where a deposit of a plant has been made in conjunction with a utility application (35 U.S.C. 101 ).
A plant patent is granted only on the entire plant. Only one claim is necessary and only one is permitted. A method claim in a plant patent application is improper. An example of a proper claim would be "A new and distinct variety of hybrid tea rose plant, substantially as illustrated and described herein."
If the drawings or photographs are in color, two color copies of each drawing or photograph are required. If the required copies of the drawings are not included, the application will be accorded a filing date, but correction will be required before the application is forwarded for examination.
*****
*****
*****
*****
*****
Form Paragraphs 16.06, 16.07, and 16.11 may be used to object to the drawing disclosure.
The disclosure is objected to under 37 CFR 1.165(b) because applicant has not provided copies of the color drawing in duplicate. Correction is required.
The disclosure is objected to under 37 CFR 1.165(a) because Fig. [1] not artistically and/or competently executed.
The disclosure is objected to under 37 CFR 1.165(a) because the drawings are of an inadequate scale to show the distinguishing features of the plant.
The applicant may be required to furnish specimens of the plant, or its flower or fruit, in a quantity and at a time in its stage of growth as may be designated, for study and inspection. Such specimens, properly packed, must be forwarded in conformity with instructions furnished to the applicant. When it is not possible to forward such specimens, plants must be made available for official inspection where grown.
Specimens of the plant variety, its flower or fruit, should not be submitted unless specifically called for by the examiner.
Form Paragraph 16.13 may be used to require specimens.
Applicant [1] required to submit [2] in accordance with 37 CFR 1.166.
Plant applications are subject to the same examination process as any other national application. As such, the statutory provisions with regard to patentable subject matter, utility, novelty, obviousness, disclosure, and claim specificity requirements apply (35 U.S.C. 101, 102, 103, and 112 ). The sole exception in terms of applicability of these statutory provisions is set forth in 35 U.S.C. 162.
The prior art considered by the examiner is developed by a search of appropriate subclasses of the United States patent classification system as well as patent and nonpatent literature data bases. Where appropriate, a report may be obtained from the Agricultural Research Service, Horticultural Research Branch, Department of Agriculture. See MPEP § 1609.
The President may by Executive order direct the Secretary of Agriculture, in accordance with the requests of the Director, for the purpose of carrying into effect the provisions of this title with respect to plants (1) to furnish available information of the Department of Agriculture, (2) to conduct through the appropriate bureau or division of the Department research upon special problems, or (3) to detail to the Director officers and employees of the Department.
Applications may be submitted by the Patent and Trademark Office to the Department of Agriculture for study and report.
The authority for submitting plant applications to the Department of Agriculture for report is given in:
Executive Order No. 5464, October 17, 1930. Facilitating the consideration of applications for plant patents.
I, Herbert Hoover, President of the United States of America, under the authority conferred upon me by act of May 23, 1930 (Public No. 245) [now 35 U.S.C. 164 ], entitled "An act to provide for plant patents," and by virtue of all other powers vested in me relating thereto, do hereby direct the Secretary of Agriculture: (1) to furnish the Commissioner of Patents such available information of the Department of Agriculture, or (2) to conduct through the appropriate bureau or division of the department such research upon special problems, or (3) to detail to the Commissioner of Patents such officers and employees of the department, as the Commissioner may request for the purpose of carrying said act into effect.
Where the examiner considers it necessary to the examination of the plant patent application, a copy of the file and drawing of the application are forwarded to the National Program Leader for Horticultural Crops, Agricultural Research Service (ARS), U.S. Department of Agriculture, along with a request for a report as to whether the plant variety disclosed is new and distinct over known plant varieties. As the report is merely advisory to the Office, it is placed in the file but is not given a paper number. The copy of the report is customarily utilized by the examiner in the preparation of his or her action on the application.
The report may embody criticisms and objections to the disclosure, may offer suggestions for correction of such, or the report may merely state that:
"Examination of the specification submitted indicates that the variety described is not identical with others with which our specialists are familiar."
The action on the application by the examiner will include all matters as provided for in other types of patent applications. See 37 CFR 1.161.
With reference to the examination of the claim, the language must be such that it is directed to the "new and distinct variety of plant." This is important as under no circumstance should the claim be directed to a new variety of flower or fruit in contradistinction to the plant bearing the flower or the tree bearing the fruit. This is in spite of the fact that it is accepted and general botanical parlance to say "A variety of apple or a variety of blackberry" to mean a variety of apple tree or a variety of blackberry plant.
Where the application is otherwise allowable, a claim which recites, for example "A new variety of apple characterized by," may be amended by the insertion of - tree - after "apple" by an examiner’s amendment.
By the same token, the title of the invention must relate to the entire plant and not to its flower or fruit, thus: Apple Tree, Rose Plant.
Care should also be exercised that the specification does not contain unwarranted advertising, for example, "the disclosed plant being grown in the XYZ Nurseries of Topeka, Kansas." It follows, also, that in the drawings any showing in the background of a plant, as a sign carrying the name of an individual, nursery, etc., is objectionable and deletion thereof is required. Nor should the specification include laudatory expressions, such as, "The rose is prettier than any other rose." Such expressions are wholly irrelevant. Where the fruit is described, statements in the specification as to the character and quality of products made from the fruit are not necessary and should be deleted.
The Office action may include so much of any report of the ARS as the examiner deems necessary, or may embody no part of it. In the event of an interview, the examiner, in his or her discretion, may show the entire report to the inventor or attorney.
Form Paragraph 16.12 may be used to reference portions of the ARS report.
This application has been submitted to the U.S. Department of Agriculture for a report. Pertinent portions follow: [1]
The report of the ARS is not in the nature of a publication and matters raised therein within the personal knowledge of the specialists of the ARS are not sufficient basis for a rejection unless it is first ascertained by the examiner that the same can be supported by affidavits by said specialists (37 CFR 1.104(d)(2) ). See Ex parte Rosenberg, 46 USPQ 393 (Bd. App. 1939).
Form Paragraphs 16.04 and 16.08, as appropriate, may be used to reject the claim.
The claim is rejected under 35 U.S.C. 102 as failing to patentably distinguish over [1].
The claim is rejected under 35 U.S.C. 112 [1] because [2].
The preparation of a plant patent application for issue involves the same procedure as for other applications (37 CFR 1.161 ), with the exception that where there are color drawings, the better one of the two judged, for example, by its sharpness or cleanliness is selected to be printed in the patent.
The International Patent Classification symbols, most recent edition, should be placed on the Issue Classification form of all plant patent applications being sent to issue.
All plant patent applications should contain an abstract when allowed.
The International Convention for the Protection of New Varieties of Plants (generally known by its French acronym as "UPOV Convention") was adopted on December 2, 1961, by a Diplomatic Conference held in Paris.
The UPOV Convention has been revised on November 10, 1972, on October 23, 1978, and on March 19, 1991, in order to reflect technological developments in plant breeding and experience acquired with the application of the UPOV Convention. As of December 5, 2012, 71 states and organizations were party to the UPOV Convention.
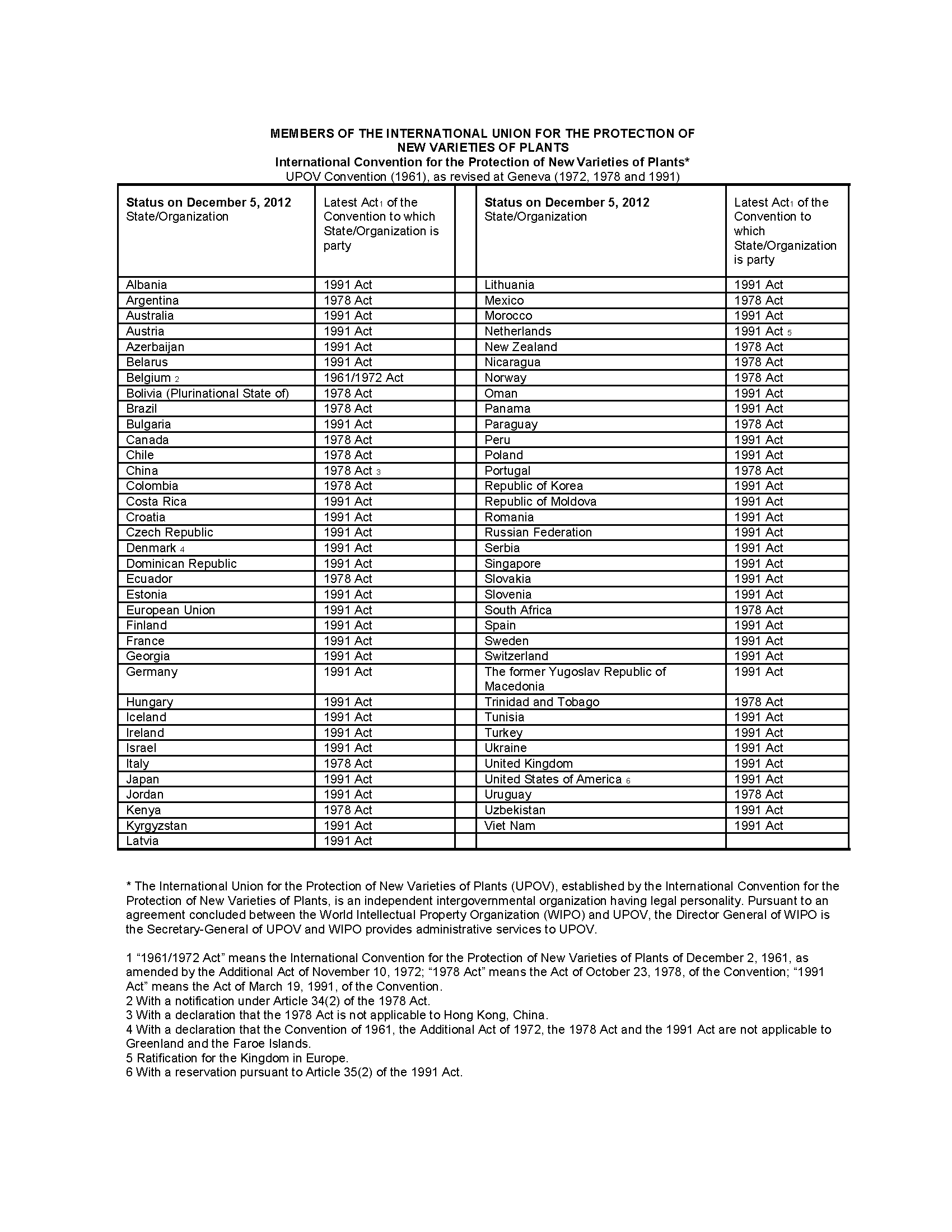
Most states adhere to either the 1978 text or the 1991 text. The United States adheres to the 1991 text, and has a reservation under Article 35(2) of the text (which allows plant patents rather than breeder’s rights certificates to be granted).
The 1961, 1978, and 1991 texts guarantee to plant breeders in each member state both national treatment and the right of priority in all other member states. In many states, new plant varieties are protected by breeders’ rights laws rather than patent laws. Accordingly, the Paris (Industrial Property) Convention cannot always be relied on to provide these and other rights.
Insofar as the patenting of asexually reproduced plants in the United States is concerned, both national treatment and the right of priority have been accorded to foreign plant breeders since enactment of the plant patent law in 1930 (now 35 U.S.C. 161 -164 ). See MPEP § 1613 for the right of priority based upon an application for plant breeder’s rights.
Application of the UPOV Convention in the United States does not affect the examination of plant patent applications, except in one instance. It is now necessary as a condition for receiving a plant patent to register a variety denomination for that plant. Inclusion of the variety denomination in the patent comprises its registration. The registration process in general terms consists of inclusion of a proposed variety denomination in the plant patent application. The examiner must evaluate the proposed denomination in light of UPOV Convention, Article 13. Basically, this Article requires that the proposed variety denomination not be identical with or confusingly similar to other names utilized in the United States or other UPOV member countries for the same or a closely related species. In addition, the proposed denomination must not mislead the average consumer as to the characteristics, value, or identity of the patented plant. Ordinarily, the denomination proposed for registration in the United States must be the same as the denomination registered in another member state of UPOV.
Pursuant to 37 CFR 1.76(b)(3), the Latin name and the variety denomination for the plant claimed may be included in an application data sheet (ADS). The Office, pursuant to the UPOV Convention, has been asked to compile a database of the plants patented and the database must include the Latin name and the variety denomination of each patented plant. Having this information in an ADS will make the process of compiling this database more efficient.
Form Paragraph 16.05 may be used to object to the disclosure as lacking a common or market name or "denomination" of the plant.
The disclosure is objected to under 37 CFR 1.121(e) because no "variety denomination" of the instant plant has been set forth in the disclosure. 37 CFR 1.163(c)(4). Correction by adding such a name is required.
The disclosure is objected to under 37 CFR 1.121(e) because the Latin name of the genus and species of the instant plant has not been set forth in the disclosure. 37 CFR 1.163(c)(4). Correction by adding such a name is required.